



















In the reaction HCl + NH₃ --> NH₄⁺ + Cl⁻ which is the conjugate acid and which is the conjugate base? Arrhenius acid, Bronsted-Lowry acid. What types of acids is HCl? Bronsted-Lowry base, not an Arrhenius base (because it has no OH in the formula) What types of bases is NH₃? OH⁻, OH⁻ What are the products of the following acid base reaction? O²⁻+H₂O--> HSO₃⁻, HPO₄² What is the conjugate base of HSO4-? SO42-What is the conjugate base of HCO3-? CO32-What is the conjugate acid of CO32-? HCO3-What is the conjugate acid of HCO3-? H2CO3. What is the conjugate acid of H20? H3O+ Double headed arrows mean _____. Weak acids/bases. What is it called when water reacts with itself? Autoionization . Majority of weak acids are in the form of what? Reactants. ALL HSO4- is a base since it has the ability to accept a proton but it is a conjugate base to H2SO4 since it is formed by the H2SO4 after donating a proton. The conjugate base of HSO4- is SO4 (2-), if that’s the question that you are asking. 26.8K views View 13 Upvoters Answer to 6. Identify the conjugate base of HSO4- A) OH- B) H2SO4 C) H20 D) H2SO3 E) SO42- 7. A 0.14 M HNO2 solution is 5.7% ioniz... What is the conjugate base of HSO4- and HC2H3O2? What is the conjugate acid of CN- and CO3(2-) Source(s): conjuguate base hso4: https://tr.im/gwKhm. 0 0. Sherry. Lv 4. 5 years ago. complex aspect. seek using yahoo or google. this might help! 0 1. How do you think about the answers? You can sign in to vote the answer. Sign in . greenwhitecollege. Lv 4. 1 decade ago. In HSO4 it is SO4 because it HSO4- + H + Now, HSO4-is a base since it has the ability to accept a proton but it is a conjugate base to H 2 SO 4 since it is formed by the H2SO4 after donating a proton. The molecule can only resonate through 3 of its Oxygens while the last one holds the hydrogen. B. Loss of a proton from an acid forms its conjugate base. C. Gain of a proton by an acid forms its conjugate base. D. Brønsted-Lowry acid-base reactions always result in the transfer of a proton from a base to an acid. Bloom's Level: 3. Apply Difficulty: Easy Gradable: automatic Section: 02.01 Subtopic: Acid/Base definitions In your equation, you've written the conjugate base of hydroxylamine. To make a conjugate acid, add an H+ ion, not take one away (and also make an OH- ion). The equilibrium sign tells you that it is a weak base. 1 0. Anonymous. 4 years ago. Honh2. Source(s): https://owly.im/a7WvA. 0 2. ellen . 6 years ago. sophisticated subject. try searching into google or bing. just that can help! 0 6. Still Write The Conjugate Acids Of The Following: CH3OH: HSO4- 2. Write The Conjugate Base Of The Following: A) H3PO4 B) CH3NH3+ 3. What Is The Useful Buffering Range For A Buffer Prepared With Formic Acid (Ka = 1.78 X 10-4) 4. Calculate The PH Of A Buffer Solution That Is 0.080 M Sodium Benzoate And 0.020 M Benzoic Acid? The PKa Of Benzoic Acid Is 4.20. Give the conjugate acid and the conjugate base for HSO4-. Answer: conjugate acid: H2SO4. conjugate base: SO42-19 Explain why AlCl3 is a Lewis acid. A Lewis acid is an electron pair acceptor. Aluminum in AlCl3 has an empty p orbital that can accommodate the pair of electrons provided by a Lewis base. 20 E) V. 21 Identify the most acidic carboxylic acid. A) ICH2COOH. B) BrCH2COOH. C) CH3COOH. D
[index] [810] [4253] [2653] [4630] [8517] [3361] [4036] [2773] [1346] [5004]
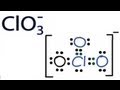
In this video we will look at the equation for HCl + H2O and write the products. When we add HCl to H2O the HCl will dissociate and break into H+ and Cl-. ... A step-by-step explanation of how to draw the SO4 2- Lewis Dot Structure (Sulfate ion).For the SO4 2- structure use the periodic table to find the total numb... A step-by-step explanation of how to draw the NO3- Lewis Structure (Nitrate Ion). Get more chemistry help at www.Breslyn.org.For the NO3- Lewis structure,... In this video we will look at the equation for H2SO4 + H2O and write the products. There are two reactions we need to consider since both of the hydrogens... This chemistry video tutorial shows you how to identify an ionic compound as acidic, basic, or a neutral salt. You need to know the 6 common strong acids su... A step-by-step explanation of how to draw the SO3 2- Lewis Structure (Sulfite Ion). For the SO3 2- Lewis structure the total number of valence electrons ... A quick explanation of the molecular geometry of ClO4 - (Perchlorate ion) including a description of the ClO4 - bond angles.Looking at the ClO4- Lewis struct... A step-by-step explanation of how to draw the ClO3- Lewis Structure (Chlorate Ion). The ClO3- Lewis structure is a good structure to help you understand w... Trick to Find Conjugate Acid and Conjugate Base / Ionic Equilibrium Tricks A step-by-step explanation of how to draw the CO3 2- Lewis Dot Structure (Carbonate ion).For the CO3 2- structure use the periodic table to find the total nu...
Copyright © 2024 m.top10casinosgame.rent